
Which Metals Dissipate Heat the Best
Some metals dissipate heat more effectively than others, and this thermal conductivity is essential in a range of applications. Thermal conductivity is the measure of a metal’s ability to conduct heat. What this means is that that the metal acts to cool temperatures, through a process of dissipation.
The metals with the highest thermal conductivity are copper and aluminium. The lowest are steel and bronze.
Metals that conduct heat effectively are used in applications where transferring heat is essential, either as part of a cooling or heating process. On the other hand, metals like steel, which is a poor conductor of heat, are suitable for high temperature environments where heat resistance is crucial.
For example, as an effective heat conductor, copper is used in heating rods and wires, hot water tanks and heat exchangers. Similarly, aluminium alloys are the most common material in heat sinks.
Where heat resistance is an essential function, then metals with a low thermal conductivity are most appropriate, for example, aeroplane engines made of steel.
In thermal conductivity applications, these metals must first be manufactured to make them suitable for their end purpose. This is why high temperature insulation and furnace safety systems are crucial for the foundry and steel industry.
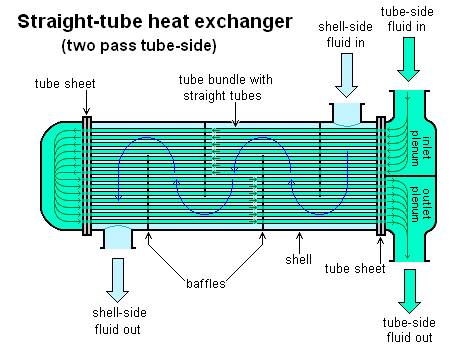
Heat Exchangers
Heat exchangers are devices that transfer heat from one form to another. This exchange of matter might be a fluid, such as oil or water, or moving air. The main metal in heat exchangers is copper, but aluminium can provide a cost-effective alternative in some applications. Both are used because they conduct heat well.
A common type of heat exchanger is the car radiator. This engine coolant is made from layers of metal sheets stacked together, with an aluminium core.
It cools the engine by circulating a liquid water or oil-based coolant. This liquid is heated through the engine block, then loses its heat through the radiator before being returned to the engine.
-Heat exchangers are also used in aircraft engines to remove excess heat, and in military equipment, lasers, x-rays and power supplies.
-Industrial facilities that use heat exchangers include nuclear power plants and chemical plants. Typically this involves copper-nickel alloy tubing, with a good resistance to corrosion.
-Gas water heat exchangers transfer heat generated by gas fuels to water in residential and commercial boilers.
-Evaporator units drive air-to-air heat exchange in air-based heat pumps used in domestic and commercial heating systems.
Heat Sinks
These are a special form of heat exchanger reliant on thermal conductivity to transfer heat generated by electronic or mechanical devices into a moving coolant fluid, which then transfers heat away to cool.
Again, these use metals with a high thermal conductivity.

Heat sinks are commonly made from aluminium alloy, which has one of the highest thermal conductivity values. They are used in semiconductors for a variety of consumer and industrial electronics.
Computers use heat sinks to cool central processing units and graphics processors, but you will also find them in power transistors and LEDs.
Perhaps a more easily recognisable application of thermal conductivity, drawing on heat dissipation qualities, is cookware. High-quality pans have copper bottoms because this will conduct the heat quickly, spreading it evenly across the surface.
Aluminium and Copper Smelting Processes
As thermally conductive metals, copper and aluminium are of huge practical value. However, the smelting process to extract these metals from their ores itself requires expert thermal management.
Induction furnaces normally process copper and aluminium, which have a high melting temperature of 1084°C and 660°C respectively. This induction heating is cleaner and more energy-efficient than traditional methods, but it requires precise temperature control and thermal management.

Induction furnaces do not have a refining capacity, so the materials they process must first be clean of any oxidation products. These furnaces can be either coreless, or have a molten metal loop winding through an iron core.
Insulation and Furnace Safety
Just as copper and aluminium are used in heat transfer, so this process aids the actual production of these metals in the first place. Microporous high temperature insulation helps prevent heat transfer in furnaces smelting these metals.
Elmelin’s microporous material is called Elmtherm, and it comes in several grades. In Aluminium launder systems it optimises movement and minimises heat loss; and in melting furnaces it helps maintain even heat distribution and the quality of the finished product.
Another aspect of copper and aluminium smelting is ensuring furnace safety. Vapourshield is especially effective in controlling emissions when melting down copper alloys which have different chemical components.
Supporting Thermal Conductivity
Elmelin supports a broad range of industries that rely on heat transfer processes using thermally conductive metals that dissipate heat. We also provide essential high temperature insulation for foundries that process these metals. For more information, please call us on +44 20 8520 2248, email sales@elmelin.com, or complete our online enquiry form. We’ll get back to you as soon as possible.
